Background: Survival rates for children and adolescent/young adults (AYAs) with relapsed or refractory acute myeloid leukemia (R/R AML) remain poor. Previous clinical trials have demonstrated that the selective inhibitor of nuclear export selinexor and the BCL2 inhibitor venetoclax are each safe and active when combined with chemotherapy in children with R/R AML (NCT02212561, NCT03194932). However, many patients are refractory to these combinations. Preclinical data indicate that selinexor and venetoclax are synergistic and that selinexor may abrogate resistance to venetoclax. We thus aimed to characterize the toxicity profile and explore preliminary activity of venetoclax and selinexor with chemotherapy in pediatric and AYA patients with R/R AML and report results from the dose escalation phase of the SELCLAX clinical trial (NCT04898894).
Methods: SELCLAX is a Phase I and expansion cohort study of selinexor and venetoclax in combination with chemotherapy in pediatric and AYA patients with R/R AML. For the dose escalation phase, eligibility criteria included < 30 years of age, adequate organ function and performance status, primary refractory, early first relapse (< 12 months from initial diagnosis), relapsed and refractory to salvage, post-transplant relapse or second or greater relapse AML or acute leukemia of ambiguous lineage (ALAL). The primary endpoint was determination of the recommended phase 2 dose (RP2D) of venetoclax and selinexor with chemotherapy. In the dose level 1 (DL1) cohort, venetoclax was given at 360 mg/m 2 per dose (max 600 mg) on days 1-21 in combination with selinexor at 40 mg/m 2 on days 1, 8, 15. For dose level 2 (DL2), venetoclax was dosed as in DL1, and selinexor was dosed at 40 mg/m 2 twice weekly on days 1, 3, 8, 10, 15, 17. Chemotherapy (fludarabine 30 mg/m 2, cytarabine 2 g/m 2 ± granulocyte colony stimulating factor 5 mcg/kg [FLA(G)]) was given on days 16-20. Response was evaluated by blast percentage in day 8 and 15 peripheral blood and in day 15 and end-cycle bone marrow. Any patient with a two-log (100-fold) reduction in measurable residual disease (MRD) between baseline and Day 15 was designated an “exceptional responder” (ER). At the discretion of the treating physician, ER patients could continue venetoclax until day 28 with additional selinexor (DL1: day 22; DL2: day 22, 24); FLA(G) could either be omitted or given on days 30-34; omission of FLA(G) deemed a patient non-evaluable (NE) for dose-limiting toxicity (DLT). Evaluable patients were assessed for DLTs, and dosing was escalated according to a rolling-six design.
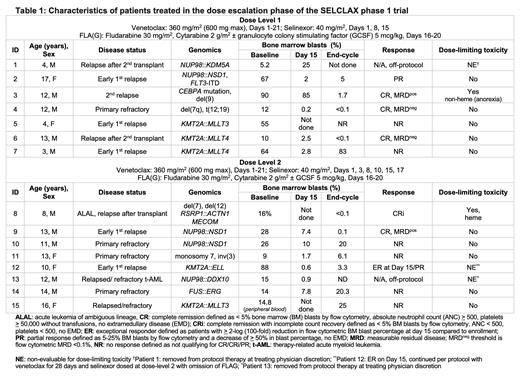
Results: As of July 14, 2023, fifteen patients (aged 3-17 years) with R/R AML (n=14) or ALAL (n=1) were enrolled in the dose escalation phase ( Table 1). Nearly all patients had high-risk cytomolecular features: KMT2A-rearranged (n=5), NUP98-rearranged (n=5), -7/del(7) (n=3), or FUS::ERG (n=1). Seven patients were enrolled on DL1 (one NE, replaced); eight patients were enrolled on DL2 (two NE, replaced). In the DL1 cohort, one DLT was reported of grade 3 anorexia requiring nasogastric feeds; however, this was cofounded by a prior history of anorexia, mucositis, and poor dentition requiring extractions and restorations. At DL2, one patient experienced a hematologic DLT with failure to recover absolute neutrophil count above 500/uL and platelet count above 25,000/uL by day 43 from start of FLA(G). There were no DLTs in the other five evaluable patients in DL2 and no serious adverse events (AEs) reported. The most common Grade 3 or 4 AE was febrile neutropenia (16.7%). Of the evaluable patients, five of twelve (41.7%) achieved complete response (CR) or complete response with incomplete count recovery (CRi). One patient was an ER on Day 15 at DL2 and did not receive FLA(G); thus, this patient was not evaluable for DLT assessment. Six patients proceeded to hematopoietic cell transplant after protocol therapy, five of whom are alive to date.
Conclusion: Venetoclax and selinexor with FLA(G) chemotherapy is a tolerable combination in pediatric and AYA patients with R/R AML. Early activity of this combination regimen was observed in some patients, including those with primary refractory or early first-relapsed AML. Enrollment of the dose expansion phase at DL2 is underway in two cohorts: venetoclax-naïve and venetoclax-exposed.
Disclosures
Karol:Jazz Pharmaceuticals: Consultancy; Servier: Consultancy. Tasian:Amgen: Other: travel support ; Aleta Biotherapeutics: Membership on an entity's Board of Directors or advisory committees; Syndax Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Beam Therapeutics: Research Funding; Incyte Corporation: Research Funding; Kura Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding. Place:Novartis: Research Funding; Servier: Research Funding; AbbVie: Research Funding; Triterpenoid Therapuetics: Current equity holder in private company. Rubnitz:Biomea, Inc: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal